For each of the molecules below determine the electron geometry – For each of the molecules below, determine the electron geometry. This guide delves into the fundamental concept of electron geometry, exploring its significance and the theories that govern it. Understanding electron geometry is crucial for comprehending the structure, properties, and reactivity of molecules.
The subsequent sections will delve into the intricacies of molecular orbital theory and VSEPR theory, providing a comprehensive understanding of how these theories predict electron geometry based on the arrangement of valence electrons. Moreover, practical applications of electron geometry in various scientific disciplines will be discussed, highlighting its importance in fields such as chemistry, biology, and materials science.
Electron Geometry
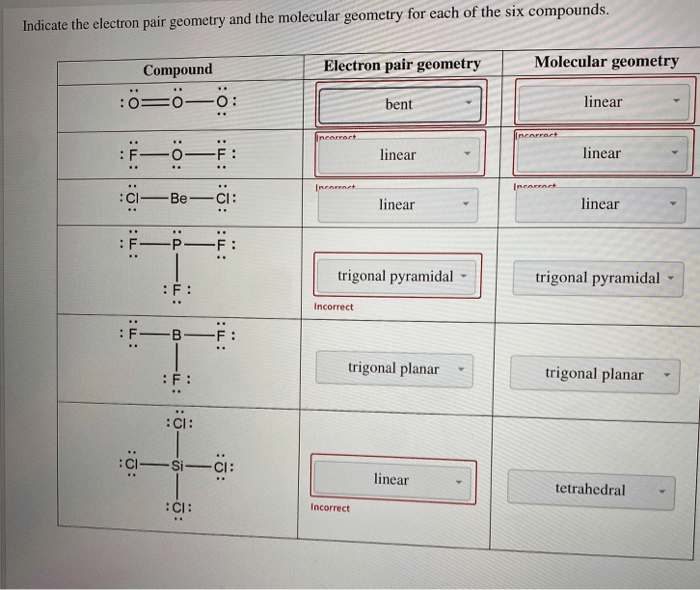
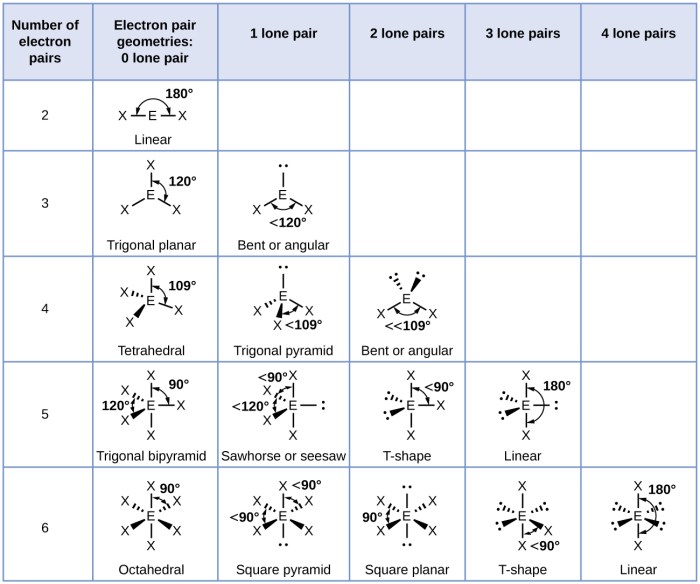
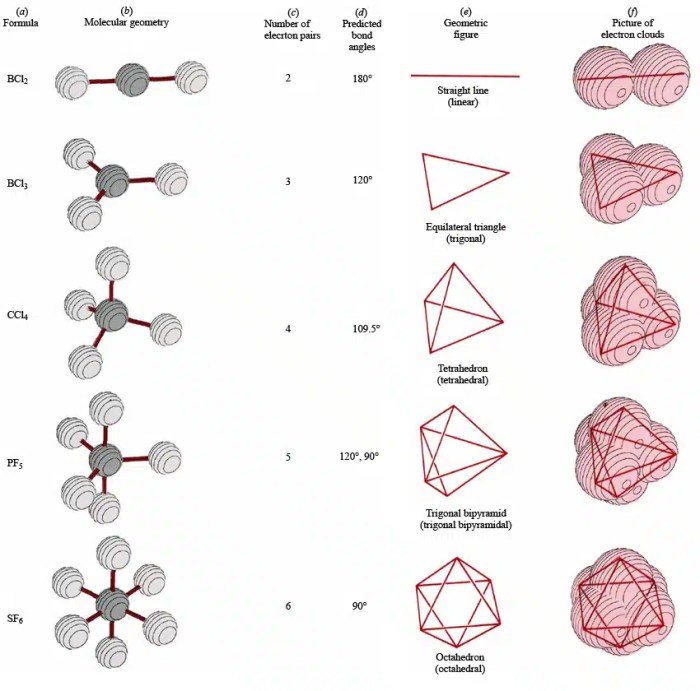
Electron geometry refers to the three-dimensional arrangement of electron pairs around an atom in a molecule. Determining electron geometry is crucial as it provides insights into molecular shape, bonding, and reactivity.
Identifying Electron Geometry
| Molecule | Lewis Structure | Electron Geometry | Molecular Shape |
|---|---|---|---|
| CH4 | :C(H)(H)(H)(H): | Tetrahedral | Tetrahedral |
| NH3 | :N(H)(H)(H): | Trigonal Pyramidal | Trigonal Pyramidal |
| H2O | :O(H)(H): | Bent | Bent |
| CO2 | :O=C=O: | Linear | Linear |
Molecular Orbital Theory
Molecular orbital theory describes how atomic orbitals combine to form molecular orbitals. The shape and symmetry of molecular orbitals determine the electron geometry and molecular shape.
VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory predicts electron geometry based on the number of valence electron pairs around the central atom. Electron pairs repel each other, resulting in specific electron geometries that minimize repulsion.
Examples, For each of the molecules below determine the electron geometry
- CH4: Tetrahedral electron geometry, tetrahedral molecular shape
- NH3: Trigonal pyramidal electron geometry, trigonal pyramidal molecular shape
- H2O: Bent electron geometry, bent molecular shape
- CO2: Linear electron geometry, linear molecular shape
Applications
Electron geometry has practical applications in chemistry, biology, and materials science. It influences molecular properties such as polarity, reactivity, and intermolecular interactions.
Questions Often Asked: For Each Of The Molecules Below Determine The Electron Geometry
What is electron geometry?
Electron geometry refers to the three-dimensional arrangement of electron pairs around an atom in a molecule.
How is electron geometry determined?
Electron geometry can be determined using molecular orbital theory or VSEPR theory, which consider the repulsion between electron pairs to predict the most stable arrangement.
What is the significance of electron geometry?
Electron geometry influences molecular shape, polarity, and reactivity, providing insights into the physical and chemical properties of molecules.