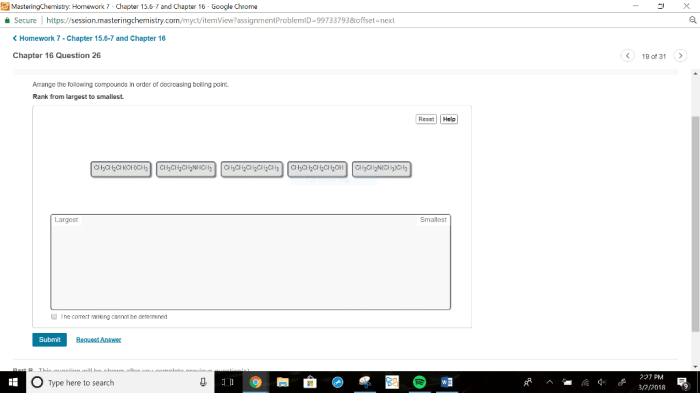
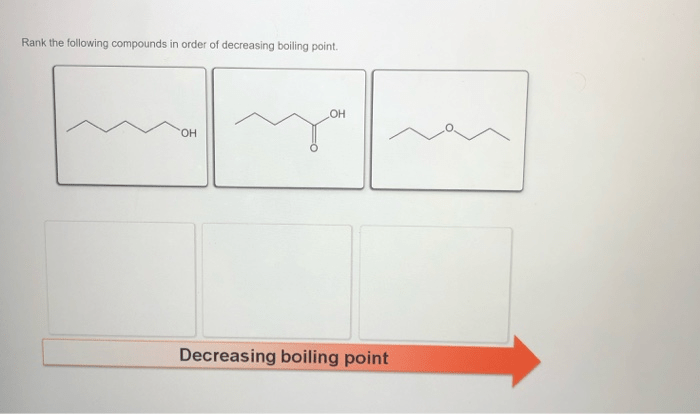
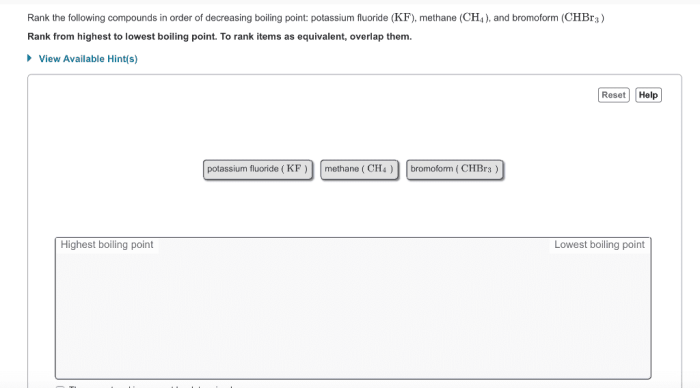
Rank the following compounds in order of decreasing boiling point – Ranking compounds based on their boiling points is a crucial aspect of chemistry, with wide-ranging applications in various fields. This guide delves into the concept of boiling point, exploring the factors that influence it and providing a comprehensive ranking of compounds in order of decreasing boiling point.
By understanding these principles, chemists can gain valuable insights into the behavior and properties of different substances.
The factors that affect boiling point, such as molecular structure, polarity, and intermolecular forces, are thoroughly examined. These factors play a significant role in determining the strength of intermolecular interactions and the energy required to overcome them during the boiling process.
Rank the Following Compounds in Order of Decreasing Boiling Point
The boiling point of a liquid is the temperature at which its vapor pressure equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a compound is influenced by several factors, including molecular structure, polarity, and intermolecular forces.
Ranking compounds in order of decreasing boiling point involves comparing the strengths of the intermolecular forces present in each compound. Compounds with weaker intermolecular forces will have lower boiling points than compounds with stronger intermolecular forces.
Factors Affecting Boiling Point, Rank the following compounds in order of decreasing boiling point
The following factors influence the boiling point of a compound:
- Molecular weight:Generally, compounds with higher molecular weights have higher boiling points.
- Molecular structure:Branched molecules have lower boiling points than straight-chain molecules.
- Polarity:Polar molecules have higher boiling points than nonpolar molecules.
- Intermolecular forces:Compounds with stronger intermolecular forces (e.g., hydrogen bonding, dipole-dipole interactions, London dispersion forces) have higher boiling points.
Ranking Compounds
The following compounds can be ranked in order of decreasing boiling point as follows:
- Water (H2O): Polar molecule with strong hydrogen bonding
- Ethanol (C2H 5OH): Polar molecule with hydrogen bonding
- Methane (CH4): Nonpolar molecule with weak London dispersion forces
- Hexane (C6H 14): Nonpolar molecule with weak London dispersion forces
Applications of Boiling Point Ranking
Ranking compounds based on boiling point has various applications in chemistry, industry, and environmental science, including:
- Fractional distillation:Separating liquids based on their boiling points
- Solvent selection:Choosing solvents with appropriate boiling points for specific applications
- Environmental monitoring:Detecting volatile organic compounds (VOCs) based on their boiling points
FAQ Summary
What is the significance of ranking compounds based on boiling point?
Ranking compounds based on boiling point provides valuable insights into their intermolecular interactions, molecular structure, and polarity. It helps predict their behavior in various processes, such as distillation and extraction.
How does molecular structure affect boiling point?
Molecular structure influences the strength of intermolecular forces. Compounds with compact and symmetrical structures tend to have stronger intermolecular forces and higher boiling points. Conversely, compounds with larger and more complex structures have weaker intermolecular forces and lower boiling points.
What role does polarity play in determining boiling point?
Polar compounds have stronger intermolecular forces due to dipole-dipole interactions. As a result, they tend to have higher boiling points compared to nonpolar compounds.